MASc candidate Shwetank Yadav uses Computational Materials Engineering to increase sustainable energy storage capacity in vehicles
[sharexy]
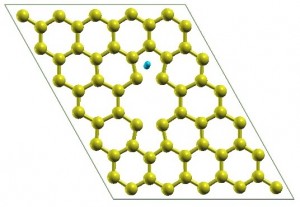
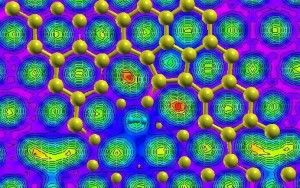
Yadav’s Computational Materials Engineering models depicting strong hydrogen adsorption in graphene defects
April 01, 2014
The quest for lightweight and efficient hydrogen storage is an ongoing challenge in energy systems design. In particular, the deployment of hydrogen to power vehicles – whose by-product is water – heavily depends on the efficiency of the storage materials itself in order to be considered viable for on-board usage.
In 2009, the United States Department of Energy (US DOE) revised its On-Board Hydrogen Storage System Targets to 5.5 wt. % hydrogen by 2015. In other words, the storage tank needs to be lightweight and efficient enough such that the hydrogen fuel is mostly used to power the vehicle, and not to carry the storage device itself.
Yadav investigated the use of controlled defects in the graphene itself in order to promote hydrogen molecule binding to the graphene surface.
In recent research – graphene, which is a one-atom thick sheet of carbon – has been studied to be one of the best nanomaterials suited to meet targeted hydrogen storage requirements. While it has been promised that metal-decorated graphene can achieve 5.5 wt. % hydrogen storage, experimental techniques have demonstrated a much lesser capability. However, a computational approach performed at the University of Toronto has proposed another way to achieve this storage capacity to an even greater extent.

Shwetank Yadav | MASc Candidate, Materials Science & Engineering
Shwetank Yadav, a U of T materials science and engineering MASc candidate in Assistant Professor Chandra Veer Singh’s Computational Materials Engineering Laboratory investigated the use of controlled defects in the graphene itself in order to promote hydrogen molecule binding to the graphene surface.
Employing a computational approach known as Density Functional Theory (DFT), Yadav’s modelling and manipulation of these controlled defects has yielded a maximum hydrogen storage capacity of 7 wt. % without the need for metal-decoration. The full work, titled “Defect Engineering of Graphene for Effective Hydrogen Storage” was published on March 26, 2014 in the International Journal of Hydrogen Energy (Volume 39, Issue 10).
“The availability of computational materials engineering has dramatically increased our ability to develop new materials by providing accurate data that was once obtained through experimental trials,” said Professor Singh. “Congratulations to Shwetank on his first publication, and to undergraduate student and second author Zhihui (Laura) Zhu as well, whose award-winning preliminary work contributed to this full publication.”