Alumnus Lyle Gordon’s discovery could lead to better understanding of tooth decay process and early detection
[sharexy]
Alumnus Lyle Gordon and his fellow researchers at Northwestern University have found that the pigmented enamel of beavers—which contains iron—is both harder and more resistant to acid than regular enamel, including that treated with fluoride (Photo: Michael Graydon)
February 13, 2015
Beavers don’t brush their teeth, and they don’t drink fluoridated water, but a new study reports beavers do have protection against tooth decay built into the chemical structure of their teeth: iron.
 |
|
| Alumnus Lyle Gordon (MSE 0T8; left) with Professor Steven Thorpe at his U of T undergraduate convocation |
|
 |
|
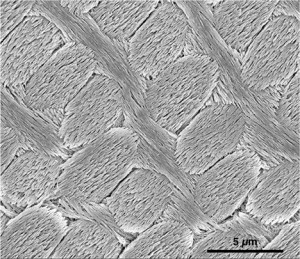
| Scanning electron microscope (SEM) image of inner enamel from the rat incisor (Photo: Lyle Gordon and Derk Joester, Northwestern University) |
Alumnus Dr. Lyle Gordon (MSE 0T8) and his PhD supervisor Derk Joester, an associate professor of materials science and engineering at Northwestern University, have found that this pigmented enamel is both harder and more resistant to acid than regular enamel, including that treated with fluoride. This discovery is among others that could lead to a better understanding of human tooth decay, earlier detection of the disease and improving on current fluoride treatments.
Layers of well-ordered hydroxylapatite “nanowires” are the core structure of enamel, but Gordon and his colleagues have discovered it is the material surrounding the nanowires, where small amounts of amorphous minerals rich in iron and magnesium are located, that controls enamel’s acid resistance and mechanical properties.
Enamel is a very complex structure, making study of it challenging. Gordon and his colleagues are the first to show unambiguously that this “amorphous,” or unstructured, phase exists in enamel, and they are the first to show its exact composition and structure.
“We have made a really big step forward in understanding the composition and structure of enamel—the tooth’s protective outer layer—at the smallest length scales,” said Joester.
“The unstructured material, which makes up only a small fraction of enamel, likely plays a role in tooth decay,” Joester said. “We found it is the minority ions—the ones that provide diversity—that really make the difference in protection. In regular enamel, it’s magnesium, and in the pigmented enamel of beaver and other rodents, it’s iron.”
The unprecedented imaging study of tooth enamel at the nanoscale will be published February 13, 2015 by the leading journal, Science.
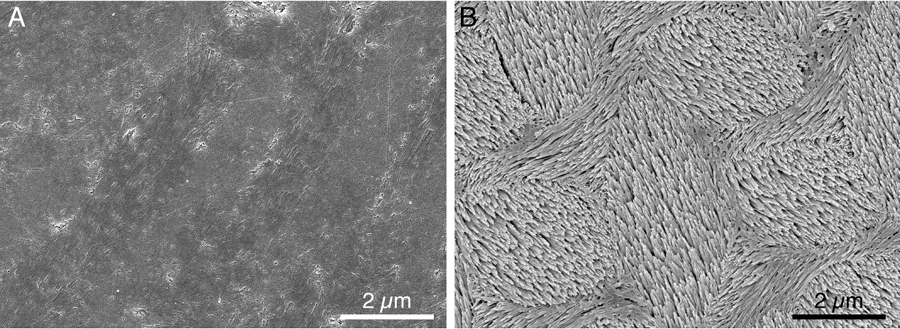
Left: smooth polished mouse enamel; right: after an acid bath, the grain boundaries dissolved quickly, leaving the crystals that make up the nanowires behind (photo: Lyle Gordon and Derk Joester, Northwestern University)
Dental caries—better known as tooth decay—is the breakdown of teeth due to bacteria. (“Caries” is Latin for “rottenness.”) It is one of the most common chronic diseases and a major public health problem, despite strides made with fluoride treatments.
According to the Canadian Dental Association, $12.5 billion a year is spent on dental services in Canada. A staggering 60 to 90 percent of children and nearly 100 percent of adults worldwide have or have had cavities, according to the World Health Organization.
In a series of experiments of rabbit, mouse, rat and beaver enamel, Gordon and his colleagues imaged the never-seen-before amorphous structure that surrounds the nanowires. They used powerful atom-probe tomography and other techniques to map enamel’s structure atom by atom. (Rodent enamel is similar to human enamel.)
The researchers next identified amorphous biominerals in the structure, such as iron and magnesium, and learned how they contribute to both the mechanical hardness and resistance of enamel to acid dissolution.The researchers subjected the teeth to acid and took images before and after acid exposure. They found the periphery of the nanowires dissolved (the amorphous material), not the nanowires themselves.
Of particular interest to Gordon and his colleagues was the pigmented enamel of the beaver’s incisors. Their studies showed it to be an improvement over fluoride-treated enamel in resisting acid. (The presence of iron gives the teeth a reddish-brown color.)
“A beaver’s teeth are chemically different from our teeth, not structurally different,” Joester said. “Biology has shown us a way to improve on our enamel. The strategy of what we call ‘grain boundary engineering’—focusing on the area surrounding the nanowires—lights the way in which we could improve our current treatment with fluoride.”
—With files from Northwestern University’s McCormick School of Engineering & Applied Science. Reproduced with permission.